
FDA Approves First CAR-T Cell Therapy for Pediatric Acute Lymphoblastic Leukemia
Tremendous progress continues to be made against the Emperor of All Maladies, cancer. One of the most exciting areas of progress involves immunotherapy, a treatment strategy that harnesses the natural ability of the body’s own immune cells to attack and kill tumor cells. A lot of extremely hard work has gone into this research, so I was thrilled to learn that the Food and Drug Administration (FDA) just announced today its first approval of a promising type of immunotherapy called CAR-T cell therapy for kids and young adults with B-cell acute lymphoblastic leukemia (ALL)—the most common childhood cancer in the U.S.
ALL is a cancer of white blood cells called lymphocytes. Its treatment with chemotherapy drugs, developed with NIH support, has transformed ALL’s prognosis in kids from often fatal to largely treatable: about 90 percent of young patients now recover. But for those for whom the treatment fails, the prognosis is grim.
In the spring of 2012, Emily Whitehead of Philipsburg, PA was one such patient. The little girl was deathly ill, and her parents were worried they’d run out of options. That’s when doctors at Children’s Hospital of Philadelphia gave Emily and her parents new hope. Carl June and his team had successfully treated three adults with their version of CAR-T cell therapy, which is grounded in initial basic research supported by NIH [1,2]. Moving forward with additional clinical tests, they treated Emily—their first pediatric patient—that April. For a while, it was touch and go, and Emily almost died. But by May 2012, her cancer was in remission. Today, five years later, 12-year-old Emily remains cancer free and is thriving. And I’ve had the great privilege of getting to know Emily and her parents over the last few years.
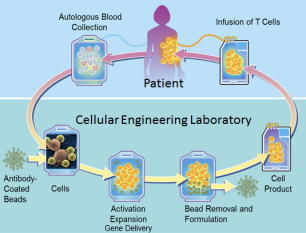
Caption: CAR-T cell therapy involves genetically engineering immune T cells to recognize specific proteins, or antigens, on tumor cells and attack them.
Credit: Carl June, University of Pennsylvania, Philadelphia
Credit: Carl June, University of Pennsylvania, Philadelphia
CAR-T cells have been called “a living drug” because doctors collect and manipulate a patient’s own immune cells to treat his or her cancer. Specifically, the harvested T cells are genetically engineered to produce new surface proteins (the CARs, or chimeric antigen receptors) that allow them to recognize and attack cancer cells more effectively. After expanding the number of these enhanced T cells, doctors infuse them back into patients to soup up their immune systems. The ability of T cells to attack a cancer cell is a sight to behold. Our ability to harness and direct that power is a true marvel of modern medicine.
This achievement in immunotherapy, though quintessentially modern, is the culmination of more than a century of scientific research. It all started back in 1890, when New York-based surgeon William Coley found a handful of patients who survived apparently fatal cancers after they suffered an acute infection. He developed a toxin—“Coley’s toxin”—that he used stimulate patients’ immune systems to treat their cancer. He believed his treatment worked. The wider medical profession was skeptical, and failure was all too common.
Over the decades, medical interest in immunotherapy waxed and waned; and researchers developed an array of immunotherapeutic interventions, to varying effect. Ultimately, a number of basic science advances in immunology, cell biology, and genetics were needed to make real progress in immunotherapy possible.
These advances came largely in the 1970s and 1980s—many with NIH support. They were recognized by visionaries, such as NIH’s own Steven Rosenberg, as providing new insights into longstanding questions about how to train our immune systems to recognize and fight cancer. Rosenberg, still at NIH’s National Cancer Institute (NCI), had been thinking about immunotherapy since the 1960s, when he witnessed the rare case of a patient with terminal cancer who appeared to be cured by a separate infection. Drawing upon new scientific knowledge, Rosenberg had early success in the 1980s with a precursor to CAR-T therapy [3]. More recently, he developed and successfully administered his own version of CAR-T [4].
With immunotherapy, as with so many of our medical interventions, the path from original discovery or observation to effective treatment is long, circuitous, and densely populated. In fact, a fascinating recent study showed that the development of another form of cancer immunotherapy, so-called “checkpoint inhibitors,” arose from the work of some 7,067 scientists, toiling over the course of a century [5]. Immunotherapy is indeed the achievement of many minds. At the same time, the commitment and passion of researchers such as June and Rosenberg—and James Allison of the University of Texas MD Anderson Cancer Center, Houston, for checkpoint inhibitors—have been essential to pushing past obstacles and skeptics, and bringing us to this remarkable moment in medicine’s history.
As it turns out, the very same year that June and his colleagues successfully treated Emily, they partnered with the Swiss-based pharmaceutical company Novartis to develop commercially a version of CAR-T therapy, now called Kymriah™ (tisagenlecleucel). Further trials showed the treatment to be extremely promising. In July, an FDA advisory committee met to consider whether to recommend approval of the therapy. With his daughter by his side, Emily’s father, Thomas, gave impassioned and eloquent testimony on CAR-T’s behalf. In the end, the committee voted unanimously to recommend that the FDA approve the treatment. And, today, the FDA followed that recommendation and gave formal approval.
Many questions must be addressed before we can herald immunotherapeutic approaches to cancer an unqualified success. There are still too many severe reactions, too many non-responses or relapses, and, potentially, a very high price tag for their widespread use, which will be truly challenging to scale up. But we’re off to a promising start.
Emily keeps smiling. Her smile gives me hope. Seeing her grow from a young girl struggling with a frightening disease into a poised young woman who looks forward to starting the 7th grade, but still spends time to be an ambassador for immunotherapy, is one of the greatest joys I’ve had as NIH Director.
I’m eager to see where our immunotherapy researchers take us next!
References:
[1] Chimeric Antigen Receptor–Modified T Cells in Chronic Lymphoid Leukemia. Porter DL, Levine BL, Kalos M, Bagg A, June CH. N Engl J Med 2011; 365:725-773.
[2] T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. Sci Transl Med. 2011 Aug 10;3(95):95ra73.
[3] Adoptive immunotherapy for cancer. Rosenberg SA. Sci Am. 1990 May;262(5):62-69.
[4] Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, Maric I, Raffeld M, Nathan DA, Lanier BJ, Morgan RA, Rosenberg SA. Blood. 2010 Nov 18;116(20):4099-40102.
[5] From scientific discovery to cures: bright stars within a galaxy. Williams RS, Lotia S, Holloway AK, Pico AR. Cell. 2015 Sep 24;163(1):21-23.
Links:
FDA approval brings first gene therapy to the United States, FDA News Release, Aug. 30, 2017
Novartis receives first ever FDA approval for a CAR-T cell therapy, Kymriah(TM) (CTL019), for children and young adults with B-cell ALL that is refractory or has relapsed at least twice, Novartis press release, Aug. 30, 2017.
CAR T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers. (National Cancer Institute/NIH)
Emily Whitehead Foundation (Philipsburg, PA)























.png)











No hay comentarios:
Publicar un comentario